Performing break-out of unhatched eggs
Tags: Hatching | Whitepaper
, 1 March 2023

Fertility, which is determined on the breeder farm and is completely independent of what takes place in the hatchery, represents the initial potential of a batch of eggs to produce chicks. Once an embryo forms, it either continues to develop, or it dies. The rate of embryo mortality varies, depending on general conditions such as the age, nutrition and health status of the breeder flock, and the quality of egg handling and incubation. It also varies according to the phase of embryo development.
In this sense, a hatchery plays the role of host, creating the right conditions for embryo development, and the coefficient HOF (hatch of fertile eggs) expresses the quality of the services the hatchery provides. Of course, only fertile eggs can develop and hatch, and their percentage in the batch limits the potential HOS (hatch of eggs set).
In practice, an HOF of 100% can never be achieved. The best batches may reach 96–97%, and over 90% is considered to be good. Surprisingly, HOF – which is the best measure of hatchery-related procedures – is frequently not the main focus of hatchery managers, and HOS, which expresses the economic efficiency of the process, tends to receive much more attention.
To determine HOF, it is of course necessary to know the fertility of a batch of eggs, which means that the eggs need to be candled. Classical candling can be used to distinguish two main categories of eggs:
- Clears: containing true infertile eggs and eggs with embryos that died in an early stage;
- Not clears: containing live embryos and dead embryos in a more advanced stage but not identified as such.
Modern technology makes it possible to detect a heartbeat, which is of course a clear symptom of life and offers a much more precise distinction. This option, although very attractive, is however not yet commonly used.
Classical light candling can be performed early, on around day 10 of incubation, or at transfer. Early candling is usually applied if the level of fertility is uncertain, for example on eggs from very young or very old flocks or flocks with fertility problems. Most large commercial hatcheries limit candling to transfer alone, when the clears can be removed (manually or using specialised machines) or – if there are not many – transferred together with the live eggs to the hatcher. The empty spaces in the hatcher baskets may then be refilled to ensure a sufficient number of eggs containing live embryos per basket. While obvious ‘bangers’ are removed prior to transfer, contaminated and rotting eggs without visible, external symptoms will often be classified as ‘not clears’ and therefore transferred to the hatcher baskets.
What do we see at hatch?
After hatch, the hatcher baskets contain chicks and the hatch debris, which includes various types of unhatched eggs and empty shells. The picture depends on whether or not candling was carried out and with which level of accuracy clears, dead embryos and bangers were removed prior to transfer. If no removal of clears by candling is performed, all of the debris will be found in the hatcher baskets. However, even if candling has been conducted, some clears are usually still found at hatch due to human error or inaccuracies in the automatic devices.
Analysis of the hatch debris provides a useful source of information in the search for improvements. However, a credible analysis must be based on credible information, and the more unhatched eggs are opened, the more reliable this information will be. On the other hand, the size of the sample must be limited to ensure that this necessary but burdensome work remains within reason.
The number of unhatched eggs in a single hatcher basket can vary from around 2–3% to 20% or more of the eggs set. In a good hatch with eggs transferred from the setter without the previous elimination of clears, it could be as low as 7% (approximately 10 eggs in a 150-egg setter tray), which actually means an HOS of 93%. However, such a good result is not standard, and a hatchery that achieves an annual average HOS of more than 85% can be classified as very good. In many hatches, therefore, the result is lower than this and the number of unhatched eggs higher.
To relate the result to the initial number of eggs set, the number of analysed baskets (or setter trays) and their capacity must be known. We need to know whether earlier candling was performed and the proportion of the load that was eliminated before transfer, as only then is it possible to express the collected numbers as a percentage of the initial load.
Break-out
The dynamics of embryo development mean that it is possible to identify the moment at which an embryo died quite precisely, within a margin of one day. This is however not always necessary in daily hatchery practice, as a hatchery manager is not an embryologist. In large, industrial hatcheries we require quick, simple, mass procedures, and a high number of analysed eggs leading to reliable information counts more than the classification details. What the hatchery manager needs to know is whether the embryo died early, in the first days of the process, in the middle of the process, or at a late stage. This is usually a sufficient accuracy to be able to identify any possible problems. These global categories can of course be fine-tuned if the observed numbers cause particular worry. For example, late mortality can be divided into late setter and hatcher mortality.
For the purpose of analysis, it is preferable to open eggs from the blunt side to classify the contents. To analyse the signs of early development, the fluid content of the egg can be tipped onto a plate, while for embryos in a more advanced stage of development, the embryos should be removed from the shell to assess their shape, size and phase of absorption of intestines and yolk sac. An additional source of information is the shell: this includes the amount of meconium, the moistness of the membrane, the height of pipping and the status of the chorioallantois.
The results of break-out analysis can be compared to a local, hatchery-specific standard based on results obtained in the past, or to the industry standard supplied by the breeding company. An example is given in the table below:
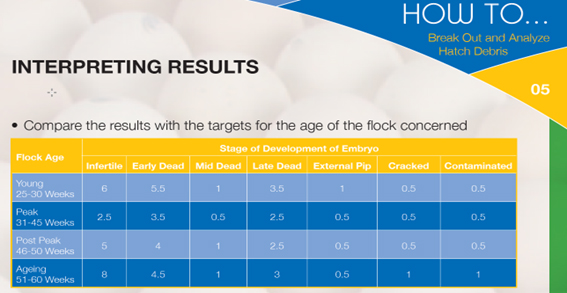
For daily hatchery practice, it is sufficient to categorise the debris into the following groups:
- True infertile – with no signs of development. The yolk of eggs kept in incubators until transfer will probably flow away, but there will be no blood, possibly with the exception of meat and blood spots that have no relation to the embryo development. There will be no membranes and the yolk will be uniformly coloured. Albumen will be watery but clear.
- Membrane mortality – this is mortality at a very early stage, and is frequently wrongly classified as infertile. A discoloured, even milky-looking egg content suggests the start of enzymatic processes, so the beginning of life. The conditions in the very early phase, even before setting, should then be checked. A very early embryonic death caused by mistakes in transport, disinfection or storage is difficult to identify at hatch. Therefore, for a clearer analysis, samples of manually selected clears at about 10 days of incubation should be taken. In the case of doubt, opening eggs before setting is suggested. The overly advanced development of the embryonic plate before setting can be one reason for membrane mortality.
- Early mortality – includes all eggs showing the first visible blood structures to ‘black eye’ (four to five days). A rate of embryonic death of around 2.5–5.5% (depending on the age and condition of the breeder flock) is normal in this period and cannot be avoided. Although many factors potentially influence early mortality – genetics, nutrition (including deficiencies and intoxication), disease, incorrect disinfection, too warm, cold or rough transport, poor storage conditions, and so on – the list of true ‘hatchery-caused mistakes’ is rather short. It includes improper egg storage, improper, unstable or non-uniform temperature in the machine and lack of turning. Humidity and ventilation issues can be practically excluded.
- Mid-age mortality (‘egg tooth, feathers’) – here we find small, bird-shaped embryos. The numbers are usually much lower than in early or late groups of embryo mortality. Possible mistakes such as over- or under-heating can accelerate or slow down embryo development but are not immediately lethal, although their effect will be seen later on. Some eggs may dry out due to poor shell quality, such as hair cracks or other mechanical damage. A potential risk is a disturbance on the critical day 14, when the embryo turns from floating on the yolk towards the long axis of the egg. Increased embryo mortality in the middle part of incubation may also suggest nutritional deficiencies or a disease in the parent stock.
- Late embryo mortality – this category usually represents the biggest share of unhatched eggs and deserves special attention. In this case, embryos have developed for a long time and reached an advanced stage, but die shortly before hatching. Their development up to this stage confirms that conditions must have been sufficient to survive so far. Late embryo mortality usually results from long-term problems related to temperature, especially after day 10 (too low, too high or fluctuating), insufficient egg weight loss or poor ventilation in the previous few days.
Temperature problems can affect the entire machine or a part of it. If the temperature was permanently too low, embryos will be small and their development retarded. In this case, many will die between day 16 and day 18; their intestines will not be absorbed and the yolk residues will be large. A long period of overheating exhausts the embryos, so that they are unable to hatch. In this case, the embryos are also small, and their internal organs are frequently not fully absorbed. Overheated embryos reaching a more advanced phase and dying in the shell tend to take a position with the head above the wing.
Problems with insufficient egg weight loss are more frequent than those related to dehydration. The air cell is small and the egg content is watery. Embryos therefore die at or before internal pipping or shortly after it, as the amount of available air in the air cell is insufficient and they simply drown inside the egg. The appearance of survivors (full bellies, sometimes in combination with red hocks) can confirm the nature of the problem.
Ventilation becomes increasingly critical after day 15. Embryos that die due to insufficient ventilation are fully developed and can die late, after day 18. The phase of absorption of the intestines and the yolk sac can help to identify when the embryo died.. Absorption of the intestines begins on day 15, followed by absorption of the yolk, which begins on day 18 and continues through day 19 until the point of internal pipping. This difficult process requires an unlimited availability of oxygen, physical power and available space for this extra load inside the body cavity. An exhausted, weak chick with a body full of watery contents cannot absorb the big yolk residue and so complete the process and properly close the navel.
Hatcher mortality is embryo mortality occurring after day 18. Although the embryos die in the hatcher, it can be caused by factors originating much earlier, either during the incubation process or related to conditions in the hatcher. Hatcher mortality is usually an effect of long-lasting problems and cannot be simply ascribed to the conditions at the moment it occurred.
When analysing the hatch debris, as well as focusing on categorising the dead embryos in groups based on developmental stage, the following observations can also be made:
- Internally pipped – opening the egg from the blunt side, we see the beak pointing up into the air cell while the eggshell is still fully closed. The timing of internal pipping can vary from chick to chick, but occurs after the intestines and the yolk sac have been absorbed and is usually on around day 19 of incubation. Mortality at this stage suggests problems that were present earlier in the setter, such as issues with temperature or insufficient development of the air cell.
- Externally pipped – external pipping follows internal pipping by about 12 hours. The shell may be perforated at just one point or partly open, and the chick inside may still be alive or it may have died. A possible reason for this may be exhaustion due to poor conditions in the setter. If many chicks are still alive in the shells, problems such as poor or non-uniform ventilation or large temperature fluctuations caused by uncontrolled humidification or a water leakage creating locally cold spots in the hatcher are more likely.
- Malposition – the embryo is ‘upside down’: opening the unhatched egg from the blunt side, we see the legs instead of the head. This is probably due to the incorrect positioning of the egg at setting. A few single embryos with this problem may not seem to be a big issue, but it should be taken seriously as setting the eggs in the wrong position reduces the hatch of the affected eggs by as much as 20–30%. Therefore, if just five eggs per setter tray are set upside down, the hatch could be reduced by almost one chick per basket, while only one unhatched egg per basket would be diagnosed as mispositioned. It is therefore recommended to check the eggs at setting. Another relatively common type of malposition is ‘head over wing’ (instead of head under the right wing), suggesting overheating in the final few days of incubation.
- Dead chicks – the presence of dead chicks in almost every hatcher basket and, in particular, a specific pattern of distribution of dead chicks in different parts of the machine, suggests ventilation problems in the hatcher. This can be caused by a misfunction of the machine or by mistakes made when adjusting the supply and exhaust air pressures.
- Deformed chicks – showing abnormalities such as brain hernia, double legs, split or crossed beaks or an open body cavity. An increased frequency in embryo abnormalities may be hereditary, and such abnormalities are more common in pure lines than in commercial cross-breeds. If seen in commercial crosses, the temperature conditions in the first few days of incubation and possible disfunctions in the turning system should be checked.
- Empty eggshells – eggshells are an important source of information. Their appearance helps to assess the general timing of the incubation process and conditions in the setter and the hatcher. Very dirty shells that are contaminated with meconium mean that the chicks were ready long before take-off, which suggests a need to correct the setting time. Another possible reason can be that the temperature set point was lowered too much while the chicks were still wet, causing them to release meconium, which is a natural response to undercooling. The height of shell pipping is related to the egg weight loss. A high egg opening over the middle part of the shell and repeated in many eggs may indicate insufficient weight loss and a need to correct the humidity profile in the incubation programme. The moisture of the membranes of the shells remaining in the hatcher baskets can also provide additional information on conditions in the hatcher. For example, shells must not be too brittle or wet, and the membranes should still be slightly flexible. Chorioallantois still visible inside the eggshell should be pale and not contain blood, which could be a sign of overheating.
- Contaminated – this category is obviously related to hygiene, which can change quickly. Dark, brownish or black egg contents in combination with an offensive smell is a sign of rotting, as long exposure to a warm environment can cause infected biological material to reach an advanced stage of decomposition. However, not all of these eggs are ‘bangers’, which are seen in every hatchery. True bangers are infected by gas-producing bacteria and tend to explode on touch, spreading contamination and a specific smell. The number of bangers is a sign of the hygiene of the entire process, from farm to hatch. The frequency of true bangers is therefore an important detail to be registered. The eggs of old flocks are particularly susceptible, because of the higher infection levels in poultry houses and worsening eggshell quality. Eggs infected by Aspergillus do not explode, but contain grey or greenish colonies of moulds, which are a warning sign for the hatchery.
- Mechanically damaged (cracked) – all egg handling presents a risk. At hatch, we may find dried-off eggs that were broken before setting, as a result of turning or transfer. By assessing the amount of remaining egg contents, we can determine when the damage occurred. Mechanical damage may be due to poor adjustment of the machines used for egg setting or transfer, eggs that are too large or lack of care by personnel, or it may simply be related to poor shell quality caused by flock-related factors such as nutrition, disease or age.
Break-out techniques
Break-out can be performed in various ways. In a mass procedure, usually applied in very large hatcheries, several people work as a team to open the unhatched eggs one by one and report the results to a ‘secretary’. The data collected is gathered in a continuous database, such as an Excel spreadsheet, which can be used to analyse trends. The break-out results collected during the different phases of the incubation process can be collated, and the resulting analysis makes it possible to identify trends and make comparisons between flocks and with breed or company standards.
If break-out is performed less systematically, a simplified method can be applied. In this case, a sufficient sample of unhatched eggs is opened and categorised on trays by type. For reliable data, the sample should be based on six to 10 hatcher baskets, representing about 1,000 eggs set. Such a physical overview of the analysed debris is worthwhile, as any category that is found to require particular attention can then be rechecked in more detail, additional questions can be formulated and a need for additional data can be expressed.
Advice
- Routinely calculate a Hatch of Fertile (HOF) coefficient.
- Make the break-out (even if a simplified method) a routine part of the hatchery programme, not just an occasional action.
- Keep it simple. Systematically collecting basic, up-to-date data is more important than collecting lots of less relevant details.
- Define your objective: a routine check or problem-solving? Adjust the system to collect data accordingly and fine-tune if necessary.
- Use your own data to develop local standards for different types of eggs, flock ages and procedures. Create a good, reliable database illustrating historical tendencies.
- Use sufficient numbers of setter trays and hatcher baskets for analysis.
- Draw conclusions based on the results of break-out in relation to the applied programmes and procedures.
- Be cautious about drawing conclusions and applying them to adjust programmes. Always back up your findings with information from other sources such as data on eggshell temperature, measured egg weight loss and the appearance of chicks.
Written by Maciej Kolańczyk
Senior Hatchery Specialist
I welcome your feedback on this article - and if you require any additional information, please don't hesitate to contact me.